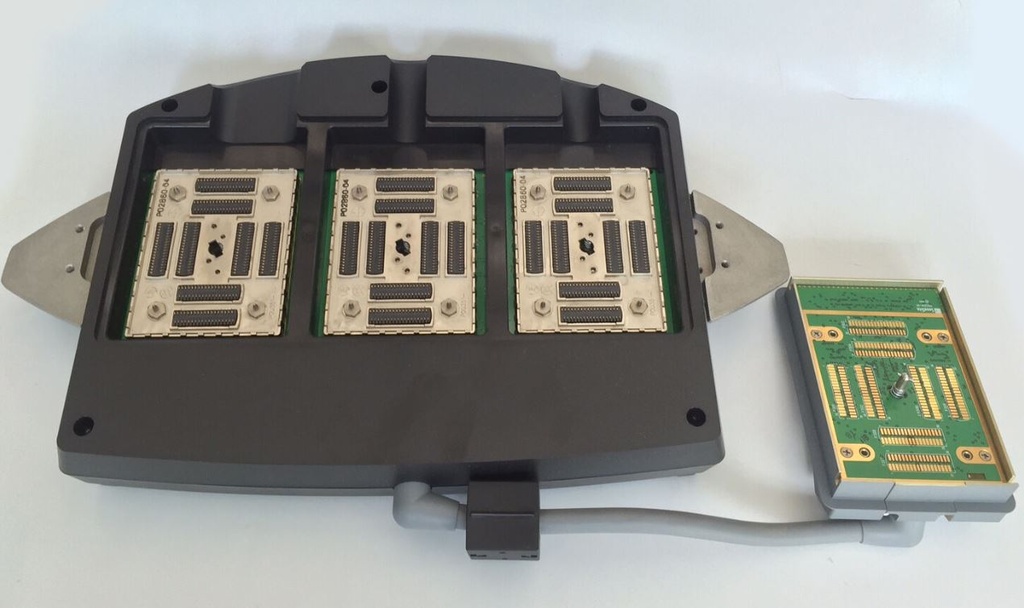
(ultrasound M-Turbo/Edge 2) TRIPLE TRANSDUCER CONNECT P16535
NST
EDIMULSA415
Valid Article
HS Code:
901812
Last Updated on:
21/09/2025, 22:01:41
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
Z11049080 - Various ultrasound instruments - hardware accessories
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes




![[EDIMULSE4--] ULTRASOUND (Sonosite M-Turbo) + TRANSDUCER C60x](/web/image/product.template/570149/image_256/%5BEDIMULSE4--%5D%20ULTRASOUND%20%28Sonosite%20M-Turbo%29%20%2B%20TRANSDUCER%20C60x?unique=1dd00df)
![[EDIMULSE8--] ULTRASOUND (Sonosite Edge 2) + TRANSDUCER rC60xi, armored c.](/web/image/product.template/578426/image_256/%5BEDIMULSE8--%5D%20ULTRASOUND%20%28Sonosite%20Edge%202%29%20%2B%20TRANSDUCER%20rC60xi%2C%20armored%20c.?unique=3af6164)
![[EDIMULSE8B-] ULTRASOUND(Sonosite Edge 2)+TRANSDUCERS rC60xi/HFL38xi/rP19x](/web/image/product.template/578427/image_256/%5BEDIMULSE8B-%5D%20ULTRASOUND%28Sonosite%20Edge%202%29%2BTRANSDUCERS%20rC60xi-HFL38xi-rP19x?unique=6f66baa)
![[EDIMULSE8BE] ULTRASOUND(Sonosite Edge 2)+TRANSD. rC60xi/HFL38xi/rP19x EN](/web/image/product.template/587335/image_256/%5BEDIMULSE8BE%5D%20ULTRASOUND%28Sonosite%20Edge%202%29%2BTRANSD.%20rC60xi-HFL38xi-rP19x%20EN?unique=4ac2722)
![[EDIMULSE8BF] ULTRASOUND(Sonosite Edge 2)+TRANSD. rC60xi/HFL38xi/rP19x FR](/web/image/product.template/587338/image_256/%5BEDIMULSE8BF%5D%20ULTRASOUND%28Sonosite%20Edge%202%29%2BTRANSD.%20rC60xi-HFL38xi-rP19x%20FR?unique=4ac2722)
![[EDIMULSE8E-] ULTRASOUND (Sonosite Edge 2)+TRANSDUCER rC60xi, armored c.EN](/web/image/product.template/587336/image_256/%5BEDIMULSE8E-%5D%20ULTRASOUND%20%28Sonosite%20Edge%202%29%2BTRANSDUCER%20rC60xi%2C%20armored%20c.EN?unique=4ac2722)
![[EDIMULSE8F-] ULTRASOUND (Sonosite Edge 2)+TRANSDUCER rC60xi, armored c.FR](/web/image/product.template/587337/image_256/%5BEDIMULSE8F-%5D%20ULTRASOUND%20%28Sonosite%20Edge%202%29%2BTRANSDUCER%20rC60xi%2C%20armored%20c.FR?unique=4ac2722)